![]() Quick Search
Quick Search
Innovating Science® - Stoichiometry in the Synthesis of an Ionic Compound
Product # IS8011
Many combinations of mono and trivalent cations yield crystals of the same stoichiometry and structure. The crystals are normally in the form of an octahedron. Sodium, potassium and ammonium ions are often the monovalent species whereas aluminum and chromium are examples of the trivalent ions. Potassium alum, KAl(SO4)2 12H2O, is the most common and is used in water purification, paper manufacturing and as a mordant in dyeing. In this experiment we will perform a series of chemical reactions which lead to the synthesis of the ionic compound potassium alum.
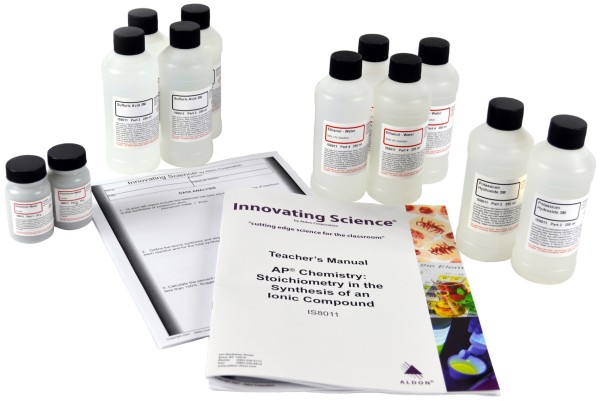
Kit Includes:
2 x 25g Aluminum Metal Powder
2 x 250mL Potassium Hydroxide, 3.0M Solution
4 x 250mL Sulfuric Acid, 3M Solution
4 x 250mL Ethanol/Water V/V 50/50% Solution
DOT Info:
UN1814, Potassium hydroxide, solution, 8, II, Ltd Qty
UN2796, Sulfuric acid, 8, II, Ltd Qty
UN1170, Ethanol, 3, III, Ltd Qty
 WARNING: This product can expose you to chemicals including Strong inorganic acid mists containing sulfuric acid and Methanol/Methyl isobutyl ketone, which are known to the State of California to cause cancer and reproductive harm. For more information go to http://www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals including Strong inorganic acid mists containing sulfuric acid and Methanol/Methyl isobutyl ketone, which are known to the State of California to cause cancer and reproductive harm. For more information go to http://www.P65Warnings.ca.gov. Innovating Science® products are For Laboratory Use Only

