![]() Quick Search
Quick Search
Innovating Science® - Small Group Learning: Oxidation-Reduction Reactions
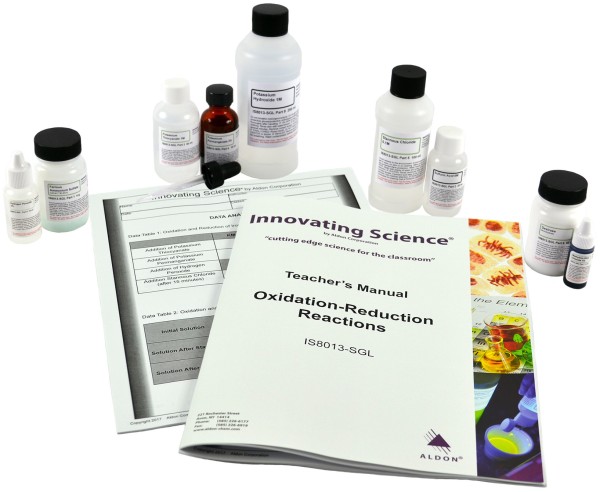
Product # IS8013-SGL
The term oxidation can mean the chemical combination of a substance with oxygen and reduction can be the removal of oxygen from a compound. When oxygen reacts with any other element (except fluorine) it acquires electrons from that element. The element that donated the electrons is said to be reduced. Three experiments will be run where a compound, which is colorless in solution when reduced, is converted to a deeply colored solution when oxidized. The complete balanced reactions for each step should be written showing the transfer of electrons during oxidation and reduction. This kit contains enough material for 5 groups. Teacher's Manual and Student Study Guide copymasters are included.
Kit Includes:
4g Ferrous Ammonium Sulfate
30mL Sulfuric Acid, 6.0M
60mL Potassium Thiocyanate, 1.0M
10mL Potassium Permanganate, 2%
10mL Hydrogen Peroxide, 3%
100mL Stannous Chloride, 0.1M
5mL Methylene Blue, 1%
250mL Potassium Hydroxide, 1.0M
50g Dextrose
DOT Info:
UN1814, Potassium hydroxide, solution, 8, PG II, Ltd Qty
UN2796, Sulfuric acid, 8, II, Ltd Qty
 WARNING: This product can expose you to chemicals including Strong inorganic acid mists containing sulfuric acid, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals including Strong inorganic acid mists containing sulfuric acid, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov. Innovating Science® products are For Laboratory Use Only

