![]() Quick Search
Quick Search
Innovating Science® - Mole Ratio of Reactants
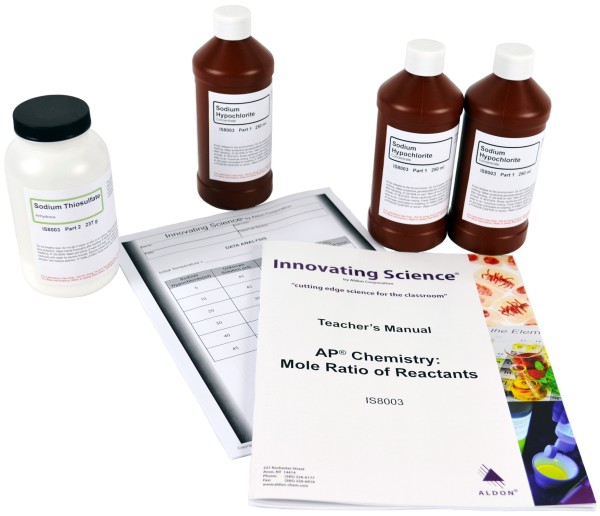
Product # IS8003
Using the method of continuous variation, two solutions are combined in various ratios. To select the ratio that produces the most product or consumes the most reactants, students must find an empirical method which is proportional to the amount of reaction that occurs. The reaction selected for this experiment is exothermic and the optimum ratio produces the greatest temperature change.
In this experiment the total numbers of moles of reactants are kept constant while varying each reactant. The measurements are made on each different ratio until the optimum ratio, the stoichiometric ratio in the equation, is made which consumes the greatest amount of reactants, produces the greatest amount of product and produces the greatest amount of heat.
Kit Includes:
3 X 250mL Sodium Hypochlorite 13% Concentrate
1 X 237g Sodium Thiosulfate, anhydrous
DOT Info:
UN1791, Hypochlorite solutions, 8, III, Ltd Qty
Innovating Science® products are For Laboratory Use Only

