![]() Quick Search
Quick Search
Innovating Science® - AP® Chemistry Lab #2: Beer's Law - Mass Percent of Copper in Brass
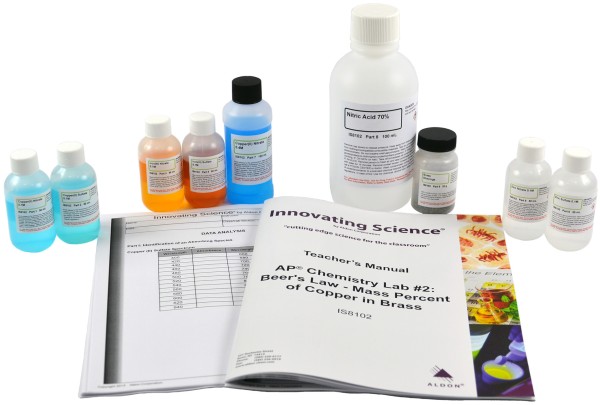
Product # IS8102
Students will design a laboratory procedure to analyze the amount of copper in brass using a spectrophotometer. Students identify the correlation among wavelength, absorbance, and concentration for each of three possible ions that may be obtained from brass: copper, zinc, and iron. The activity contains enough materials for 15 groups of students as well as a Teacher's Guide and Student Study Guide Copymasters. This lab meets Big Idea 1, Investigation 2, and Primary Learning Objective 1.16.
Kit Includes:
50mL Cupric Nitrate 0.1M
50mL Cupric Sulfate 0.1M
50mL Ferric(III) Nitrate 0.1M
50mL Ferric Sulfate 0.1M
50mL Zinc Nitrate 0.1M
50mL Zinc Sulfate 0.1M
100mL Cupric Nitrate 0.4M
100mL Nitric Acid 70%
20g Brass Pellets
Materials needed but not supplied:
Beakers, 50mL
Graduated Cylinders, 50mL
Pipets
Pipet Bulbs
Volumetric Flask, 100ml
Test Tube Racks
Watch Glasses
Test Tubes
Wash Bottle
96-well Reaction Plates
Distilled Water
Spectrophotometer w/ Cuvettes
Electronic Balance
DOT Info:
UN2031, Nitric acid, 8(5.1), II
 WARNING: This product can expose you to chemicals including Strong inorganic acid mists containing sulfuric acid, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals including Strong inorganic acid mists containing sulfuric acid, which are known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov. Innovating Science® products are For Laboratory Use Only

