![]() Quick Search
Quick Search
Innovating Science® - AP® Chemistry Lab #10: How Long Will That Marble Statue Last?
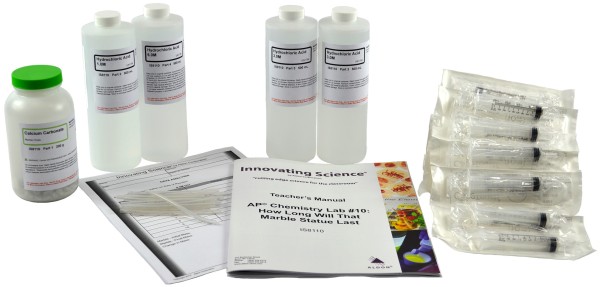
Product # IS8110
Students will observe and measure the evolution of carbon dioxide gas from the decomposition of calcium carbonate when mixed with an acid. Students will also create experiments to determine the rate of reaction with different concentrations of hydrochloric acid. The activity contains enough materials for 15 groups of students as well as a Teacher's Guide and Student Study Guide Copymasters. Meets Big Idea 4, Investigation 10, Primary Learning Objective 4.1.
Kit Includes:
1 x 200g Marble Chips
1 x 500mL Hydrochloric Acid, 1M
2 x 500mL Hydrochloric Acid, 3M
1 x 500mL Hydrochloric Acid, 6M
15 pieces Silicon Tubing
15 Dispensers,10mL
Materials needed but not supplied:
Balance, 0.001g Precision (shared)
Water, Distilled
Timer or Stop Watch
Erlenmeyer Flasks
#2 Rubber Stoppes, One Hole
Glass Tubing, 3 - 4cm
Graduated Cylinders, 25mL
Laboratory Stands
Burette Clamps or 3-prong Clamps
DOT Info:
UN1789, Hydrochloric acid, 8, III, Ltd Qty
 WARNING: This product can expose you to chemicals including Respirable crystalline silica, which is known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov.
WARNING: This product can expose you to chemicals including Respirable crystalline silica, which is known to the State of California to cause cancer. For more information go to http://www.P65Warnings.ca.gov. Innovating Science® products are For Laboratory Use Only

