![]() Quick Search
Quick Search
Innovating Science® - American Chemical Society: CO2 To the Rescue Lab Activity
Product # IS2553
This series of activities, developed by the American Chemical Society through grants from the National Science Foundation and National Institutes of Health, is comprised of a lesson that begins with a design challenge: to invent a small device that could rescue a cell phone that accidentally falls into water. The teacher starts off by showing students a balloon that inflates with carbon dioxide gas as chemicals inside the balloon react with one another. Students conduct a pair of chemical reactions to determine which of two acids react with baking soda to produce the most carbon dioxide gas. Once students determine the best acid to use, they compare the amount of gas produced with different amounts of baking soda. Finally, trying to use the smallest volumes possible, students discover how much of each reactant is needed to fully inflate a small zip-closing plastic bag to see if it can get a model clay cell phone to float.
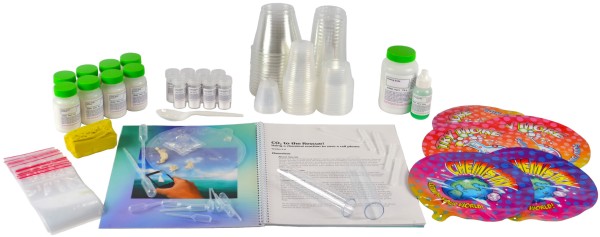
Kit Includes:
1 x 175g Baking Soda
8 x 25g Citric Acid
8 x 5g Calcium Phosphate
1 x 25mL Liquid Dish Detergent
8 Small Metric Measuring Cups
16 Portion Cups, 2oz
8 Clear 10oz Plastic Cup
16 Clear 3oz Plastic Cups
16 Clear 9oz Plastic Wide Cups
9 Long Pipettes
8 Short Pipettes
24 Small Scoops
16 Tubes
40 Reclosable Bags
1 Plastic Spoon
5 Self-inflating Reaction Bags
1 Clear Self-inflating Reaction Bag
160g Clay
WARNING: CHOKING HAZARD
children under 8 yrs. can choke or suffocate on uninflated or broken balloons. Adult supervision required. Keep uninflated balloons from children. Discard broken balloons at once.
DOT Info:
Non-regulated
Innovating Science® products are For Laboratory Use Only

